I have found two things the younger students struggle to conceptualize about an atom:
Students perceive that an atom is an atom – they are all one in the same; and they struggle to conceptualize the massive amount of space between the nucleus and electrons. I offer some ways to help students visualize/conceptualize the atom more accurately
The basics
Atoms have a core (nucleus) that is made up of protons and neutrons. The protons carry a charge, the neutrons are neutral – no charge. Surrounding the nucleus is a cloud of charged particles known as electrons. Electrons have charge that is opposite to the charge on the proton.
Our understanding of the atom today went through a few iterations over a few decades. In 1897, J.J. Thomson was the first to experimentally determine the existence of the electron, but his model of the atom was still based on limited data and understanding, and consequently wrong. In his model, he had the charged particles (electrons and protons) all mixed in together in something akin to a charged soup, or as it was dubbed, a “plum pudding”. See Figure 1. Later we learned that an atom was mostly empty space, which is the knowledge that led us to our current understanding of the atom that has a nucleus where the protons and neutrons sit. Buzzing randomly around the nucleus in discrete energy levels are the electrons, with lots of space between the electrons and the nucleus.
The number of protons an atom has will determine what element it is. For example, Hydrogen has one proton, Helium has two, Lithium has three…and so on. The atom is most stable when it has the same number of protons and electrons that give it a neutral charge. Some atoms will lose or donate an electron and become positive. Others can gain an electron and become negative. Charged atoms are called ions. Oppositely charged ions can form bonds with each other. For example, Na+ (sodium) and Cl- (Chloride) when bonded make salt (NaCl).
Now onto conceptualizing the space thing and that atoms are many and varied.
We are mostly empty space
I have found that this massive space between the electron and nucleus can be hard for younger students to conceptualize. Therefore, I have a favour to ask. Please test the following analogy and let me know how well it enables students to conceptualize the atomic structure:
Imagine a ping pong ball in the centre of a football ground (eg the MCG, if you live in Melbourne). Inside the hollow ping pong ball are all the atom’s protons and neutrons. Out in the grandstand where the crowd sit is the cloud of electrons randomly buzzing around the outside of the ground. The electrons are tiny – about 1800 times smaller than the proton, which will represent a pinprick-sized particle buzzing around the grandstand. This is a rough approximation of the distance between the nucleus and electrons and why atoms and therefore everything is mostly empty space. For the more advanced students, think about each grandstand tier representing a distinct energy level or shell that the electrons are confined to as they move randomly though the tier that corresponds to their given energy level. The caveat here is that the football ground analogy implies the atom is 2D. The electrons are not actually confined to randomly whizzing around the tier on one plane. You actually have to think of it as a ball within a ball. Telling students that we are mostly empty space bends their mind a bit, but I think it is a great thing for them to ponder about the nature of the universe.
Embrace the Periodic table and let them build (health warning!)
It is anecdote after just one pilot with Year 6 students, but I found what helped enable students to conceptualize the atom – at least the structure of it – was to get students to examine a periodic table and select an atom from it then construct a model of that atom from lollies – three different types – one for protons, one for electrons and one type for neutrons. Hence the health warning – one for the model, one for me, one for the model, one ….
I used tooth picks and bamboo skewers and got models like the one below.
 The basics of the periodic table are easy for primary kids to understand – all they really have to know is the atomic number, which will give you the number of protons. For example, assuming a neutral, stable atom, carbon has the atomic number is 6, which means there are 6 protons and therefore 6 electrons and neutrons.
The basics of the periodic table are easy for primary kids to understand – all they really have to know is the atomic number, which will give you the number of protons. For example, assuming a neutral, stable atom, carbon has the atomic number is 6, which means there are 6 protons and therefore 6 electrons and neutrons.
With each student selecting different atoms, building the models enabled students to conceptualize more accurately the structure of the atom and that atoms have different shapes and sizes – that gold atoms make gold, an iron atom makes iron and that an oxygen atom is different from a helium atom. There was this realization that everything is made of atoms, but the type of atoms determines what you can feel, see, touch, etc. Note, I would give the students an upper limit for the atomic number otherwise you will need lots of lollies.
More activities to help student learn about atoms can be found in the FLEET Schools resource, Conductors, insulators, electricity.
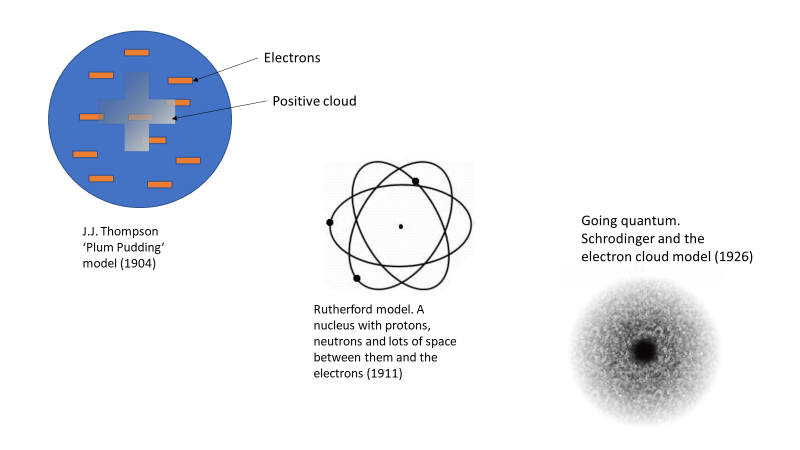
Figure 1. Atomic models starting from J.J. Thompson’s plum Pudding in 1904 through to Schrodinger’s electron cloud in 1926.
There are loads of images on the web of the different atomics models as they were developed over time. Here is one
So how big (or small) is an atom?
We need to think in nanometres. A nanometre is one billionth of a metre – or about 50,000-100,000 times thinner than a human hair. Different atoms are different sizes, but an atom is about 0.1-0.5 nanometres. Or you would need about 10 hydrogen atoms next to each other to stretch 1 nanometre. Suffice to say an atom is tiny. Today we have the technology to view and manipulate single atoms and build stuff at the atomic level. Researchers can pick up, manipulate and use atoms like Lego bricks to build novel materials and tiny new technologies. Learn more about what a nanometre is here