Making snowflakes, the science of ice and freezing, and festive-like experiments that you can try at home.
OK, we don’t normally get snow in Australia at Christmas, but we do like to put ice in our drinks on the hot Summer days, so why not talk about the snow and ice. Afterall, Winter is coming…
But here’s the thing, we know water freezes to become ice, but we don’t always have a good understanding of the why and how – at least not at the molecular level. With the following questions we have different levels of understanding. Students do, however, get to examine the beauty of math (geometry), do a bit of chemistry and, should they choose, generally make a mess of the kitchen. But we will also leave you with some things to ponder about ice.
Festivey questions
How many sides does a snowflake have and why?
Hot or cold water – what will freeze faster?
The snowflake
Snowflakes are the results of a weird combination of order and disorder. There are no two snowflakes alike, apparently, but you could say that about anything with some level of complexity – trees, rocks, identical twins…
All snowflakes (real ones anyway) have 6 sides (hexagonal) and all are symmetrical, but they are created in chaos and disorder, which is responsible for their diversity and beauty.
The disorder bit occurs in the clouds and it is the infinite variations in temperature and humidity that a snowflake is exposed to as it moves around a cloud collecting microscopic water droplets that produces its unique shape – but always hexagonal and symmetrical – the order bit.
Why the six-fold radial symmetry (hexagons)? Hexagons are the most efficient shape to pack things. Think of bees’ honeycomb. Check Figure 1 below where 6 identical badges will fit around the middle 7th badge to form a hexagon.
Figure 1. Hexagons, found everywhere in nature and a shape that enables efficient packing of stuff into a space
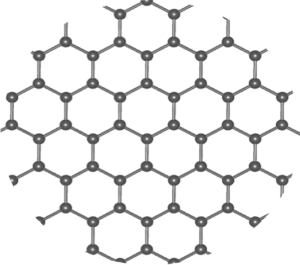
At the molecular level, water molecules are polar, where the oxygen is more negative and the hydrogen more positive. (See FLEET Schools’ Water Bender activity for a good explanation of this.) The attraction of the positive hydrogen of one water molecule to the negative oxygen on another water molecule creates a bond between the water molecules that, as they freeze and lock together, form a hexagonal crystal lattice, the same as carbon atoms in a sheet of graphene – see Figure 2.
Figure 2. Graphene showing the carbon-carbon bonds that form the hexagonal lattice. Water molecules form a similar lattice when frozen.
Look at where else can you find hexagons in nature? You will find them everywhere. A cool example is the compound eye of many insects. Especially cool are those of the dragon fly.
Make-a-flake
To start the formation of a snowflake, water vapour must condense onto a particle of something like dust or pollen and then freeze. This triggers the process of snowflaking (not sure if snowflaking is a real term, but I am owning it). Once that water vapour has frozen on the particle, other water droplets adhere to the frozen surface until you get a hexagonal seed crystal. The hexagonal shape of the seed crystal means water molecules will tend to stick to and grow out from the pointy bits of the hexagon rather than the flat surfaces. This produces the beautiful dendritc snowflakes you see in Figure 3.
 But you can also get snowflakes shaped like needles and bullets (still hexagonal). Different temperatures and humidity determine what shape the snowflake will become. See Cal Tech’s images and descriptions of the different forms.
But you can also get snowflakes shaped like needles and bullets (still hexagonal). Different temperatures and humidity determine what shape the snowflake will become. See Cal Tech’s images and descriptions of the different forms.
Figure 3. Dendritic snowflake showing the six-fold radial symmetry.
(Image: By Alexey Kljatov – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=106384255)
There are loads of videos out there that examine how snow flakes form, but I think Veritasium does one of the better jobs.
Hot or cold water. Which will freeze faster?
An initial question is at what temperature does water freeze? The answer is, it depends. The standard melting/freezing point for water is 0 degrees Celsius. But there is a phenomenon known as supercooling and now we can push the freezing point of water down to -44 degrees Celsius
But on with our question of whether hot or cold water will cool and freeze faster.
Answer – If conditions are correct then the hot one will cool faster and freeze. But what those conditions are remains one of the mysteries of science. It is a phenomenon known as the Mpemba effect named after a 13-year old Tanzanian student who noticed that ice cream made with warm milk froze faster than samples made with cold milk.
Recent modelling by some Spanish researchers suggests the Mpemba effect can be explained if you consider that not all water molecules are cooling (or heating) at the same rate. That is, the water molecules in hot water will be bouncing around, colliding with each other a lot faster than cold water molecules, but the important point according to the researchers is the need to consider that not all of those hot water molecules will be zipping around at the same speed. Some will zip; others will move relatively sedately. If the model is correct then these really slow and really fast moving molecules are crucial to working out the rate of cooling. But it is a model and the phenomenon is hard to replicate experimentally in a home science experiment. But it could be a cool classroom experiment where you can get lots of students to try it and record their methods. Does anyone show that the hot sample freezes first? Do the results let you say anything definitive about what is happening?
Potential method
Take two identical containers. Fill one with hot water from the kettle. Fill the other with cold tap water. (Actually, don’t fill them to the top because ice expands as it freezes and it could get messy.) But make sure you have the same amount of water in each container.
Put the containers in the freezer. Check which freezes first.
Variables could include different temperatures for the hotter and cooler water, temperature of the freezer, volume of water, shape of the vessels holding the water, tap water versus rain water, versus distilled water, etc. Record the different methods and freezing times. Compare the class results and see what you find.
The uncertainty of the Mpemba effect is outlined by the SciShow crew in the video below.
Or for a more artistic effect of the effect check this YouTube clip
FLEET Home Science fun
Making Crystals. (Snowfakes?)
OK, we are not making real snowflakes, but these are fun activities that can be done at home. Try using water colour paints instead of food dye in the liquid and check the difference.
Hot ice might require some adult supervision – hot water involved.
Violent crumble: And because I mention honeycomb (which is what you make here) as one of nature’s examples of a hexagon and bubbles will often form hexagons when bunched together, here is a fun and edible home science experiment. I have not checked this, but if anyone makes this, break the honeycomb and examine it under a magnifying glass and see if the holes in the honeycomb are hexagonal-shaped. Let me know.
Merry holidays everyone. Have fun